The ability of concrete to withstand the conditions for which it is designed without deterioration for a long period of years is known as durability. Durability of concrete is determined by its ability to resist weathering action, chemical attack, abrasion, or any other process of deterioration, and will retain its original form, quality, and serviceability when exposed to its environment. Durable concrete is a result of proper design, proportioning, placement, finishing, testing, inspection, curing, and continue regular inspection during services.
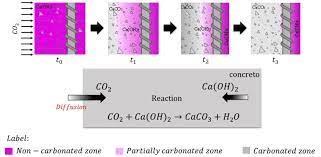
Carbonation
Concrete is an alkaline (pH>12) and this high alkalinity protects the steel from oxygen and water by forming a thin oxide layer on the steel, thus preventing the metal atoms from corroding. This protection is known as the passive layer. Carbonation is the reaction of carbon dioxide in the environment with the calcium hydroxide in the cement paste. This reaction produces calcium carbonate and lowers the pH to around 9. At this value the protective oxide layer surrounding the reinforcing steel breaks down and corrosion becomes possible. This test allows the measurement of depth of carbonation through the surface of concrete. During the test, dust samples of concrete are obtained by drilling from different depth levels. A phenolphthalein test issued to detect the loss of alkalinity associated with carbonation. Test is in accordance with EN 13295 for measurement of the depth of carbonation.

Alkali-Aggregate Reaction
In most concrete, aggregates are more or less chemically inert. However, some aggregates react with the alkali hydroxides in concrete, causing expansion and cracking over a period of many years. This alkali-aggregate reaction has two forms: alkali-silica reaction (ASR) and alkali-carbonate reaction (ACR).
Alkali-Silica Reaction (ASR) is an expansive reaction between certain forms of silica in aggregates and potassium and sodium alkalis in cement paste. The reactivity is potentially harmful only when it produces significant expansion. Indications of the presence of alkali-aggregate reactivity may be a network of cracks, closed or spalling joints, or movement of portions of a structure.

External Sulfate Attack
This is the more common type and typically occurs where water containing dissolved sulfate penetrates the concrete. A fairly well-defined reaction front can often be seen in polished sections; ahead of the front the concrete is normal, or near normal. Behind the reaction front, the composition and microstructure of the concrete will have changed. These changes may vary in type or severity but commonly include: Extensive cracking, Expansion, or Loss of bond between the cement paste and aggregate. Alteration of paste composition, with monosulfate phase converting to ettringite and, in later stages, gypsum formation. The necessary additional calcium is provided by the calcium hydroxide and calcium silicate hydrate in the cement paste. The effect of these changes is an overall loss of concrete strength.
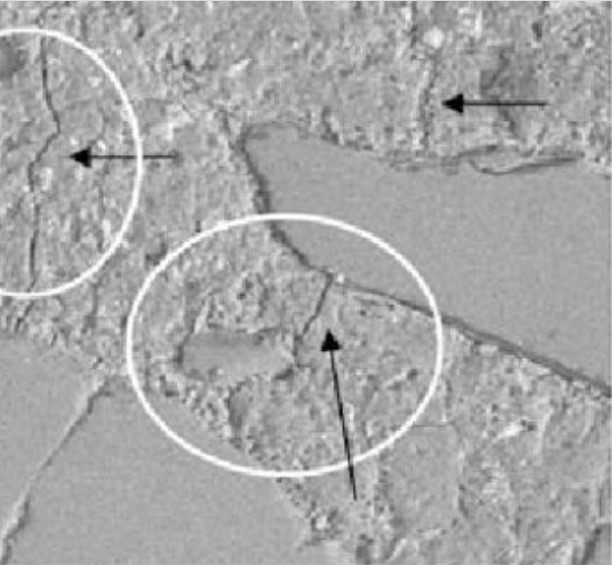
Delayed Ettringite Formation
Delayed ettringite formation (DEF) has been a significant problem in many countries. It occurs in concrete which has been cured at elevated temperatures, for example, where steam curing has been used. It was originally identified in steam-cured concrete railway sleepers (railroad ties). It can also occur in large concrete pours where the heat of hydration has resulted in high temperatures within the concrete. DEF causes expansion of the concrete due to ettringite formation within the paste and can cause serious damage to concrete structures. DEF is not usually due to excess sulfate in the cement, or from sources other than the cement in the concrete. Although excess sulfate in the cement would be likely to increase expansion due to DEF, it can occur at normal levels of cement sulfate.

Reinforcement Corrosion
The chemical and electrothermal reaction included by environmental circumstances over a long period which causes the reinforcement corrosion severely affects concrete strength and durability. The chemical reaction happens when two or more materials get in contact, which have distinct surface characteristics such as electric conduit and reinforcement, forming concentrated cells. Those concentrated cells will be formed near reinforcement bars due to the differences in the concentrated, dissolved ions like salt, acid, and chloride. It creates an oxide layer over the reinforcement. The process acts like an electric battery plus and minus point when steel rebars are used in concrete. The one end becomes the anode, and the other end becomes the cathode. Then the electrothermal reaction happens in the electrolytic cell. The iron cells lose the electrons and pass on the concrete and turn as Ferrous ions in Anode reaction and the remaining electrons are mixed with water and oxygen in Cathode reaction. Finally, an oxide layer is formed on the reinforcement and it becomes rust for over a long period. At last, the corrosion affects concrete strength and causes cracks and damages.

Freezing and Thawing
The most potentially destructive weathering factor is freezing and thawing while the concrete is wet, particularly in the presence of deicing chemicals. Deterioration is caused by the freezing of water and subsequent expansion in the paste, the aggregate particles, or both. Freeze-thaw cycles can occur daily in many climates where the temperature drops below freezing at night and rises above freezing during the day. Once the crack has expanded, and the ice has melted, more water can fill up the gap. Since the crack is bigger, it can hold more water this time around. Once the temperature drops again, the new water freezes and the crack gets nine percent larger than before. Each time this cycle occurs, the crack gets bigger and bigger.
Sewage Pipe Corrosion
In the piping network, sewage pipes behave differently in different corrosion environments. Some sewage pipes are made out of concrete and are normally heavy. Concrete pipes are highly vulnerable to moisture condensation and acidic gases. They can show a single penetration failure because of internal stresses. Sewage is a liquid-carried waste, in solution or suspension. The presence of hydrogen sulfide gas in the sewer is the primary cause of this pipe corrosion. This hydrogen sulfide produced from sulfates is found in both raw water sources and in water treatment plants. All the accumulated sulfates turn into sulfide in low-oxygen environments, and finally form corrosive sulfuric acid through bacterial action, which causes corrosion in the crown of the pipe. This corrosion is also known as “crown corrosion”. When sewage pipe material comes into contact with sulfuric acid, the pipe material corrodes, ultimately causing failure.